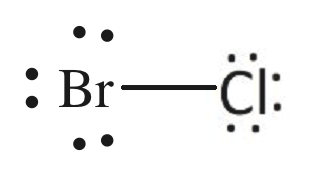BrCl is a golden yellow gas at room temperature. It is very reactive and unstable. Bromine monochloride’s boiling and melting points are 5°C and -66°C, respectively. It has an irritating odor. It is soluble in organic solvents like ether and CS2, and water. Its density is 2.32 g/mL It is a strong oxidizing agent. It is used as an industrial disinfectant. It also finds its applications in analytical chemistry to determine low Hg levels.
At room temperature, it decomposes to form bromine and chlorine gases. BrCl is a covalent compound as the constituents are halogens and share one electron to form a covalent bond. This article will discuss the procedure to find the Lewis structure, shape, hybridization, and polarity of BrCl. Let us check the below subheadings for studying its lewis structure, geometry, polarity, and hybridization.
BrCl Lewis Structure
Lewis structure or electron dot structure represents the type of bond and arrangement of constituent atoms with respect to each other. These structures can also be referred to as electronic structures. A dot represents a valence electron, and two dots (two electrons) can form a bond. The best Lewis structure is the one in which octet rule and formal charges are satisfied. • Octet Rule – It is valid for main group elements. The elements make bonds so that all the atoms in a molecule have eight valence electrons. This is because they imitate the noble gases, which are considered inert and stable. To have noble gas-like characteristics, they attain a configuration similar to that of a noble gas. • Formal Charge – In a neutral molecule, the net charge is zero. Even if equal and opposite charges are present on atoms of a molecule, it is possible for a molecule to be neutral. It compares valence electrons around an isolated atom versus the valence electrons around a bonded atom.
Steps to Draw Lewis Structure of BrCl
Step 1. Calculate the total number of valence electrons in the molecule. It is calculated by adding up the valence electrons on all atoms. In the case of a cation, we subtract the charge from the sum, and for anion, we add the charge to the sum. Elemental Lewis dot structures are shown.
Step 2. Choose the central atom. For a diatomic molecule, there is no concept of a central atom. A commonly less electronegative element is chosen as the central atom. Step 3. Draw a skeletal diagram of the molecule. A skeletal diagram is drawn in which elemental symbols are arranged.
Step 4. Arrange the valence electrons around individual atoms. The total valence shell electrons of the molecules (calculated in step 1) are placed according to the preferred bond formation. Elemental Lewis dot structures are drawn.
Step 5. Complete the octet of atoms by forming bonds. Initially, a single bond is formed between two atoms.
Chlorine and bromine atoms have attained octet configuration. Step 6. Calculate the formal charge on all atoms. The formal charge is calculated using the formula.
The lewis structure so formed is best as both the octet rule and formal charges are satisfied. The molecule BrCl3 is also made up of the same atoms. You must also read out the article on the lewis structure on BrCl3. For a better understanding, one can go through this video.
Geometry
Lewis dot structure is one of the primary and oldest methods to determine the bonding and arrangement of atoms in a molecule. Sometimes, it can help in predicting shape but not always. It fails to tell us about the exact geometry and shape of a compound, especially for polyatomic compounds. Geometry is a 3D arrangement of atoms and bonds. The shape can be the same as geometry or different from it. A diatomic molecule is always linear because any two atoms in space can only form a linear compound. The nature of two atoms does not matter. For diatomic molecules, lone pairs cannot distort the shape. Even if they try to distort, the resulting shape comes out to be linear. The shape and geometry of other simple covalent compounds can be determined using the VSEPR theory. VSEPR theory is the Valence electron pair repulsion theory. According to VSEPR theory- • The valence shell electron pairs of atoms in a molecule repel each other, and this leads to instability. • Repulsions have to be decreased to make the arrangement stable. • Electrons align themselves in such a way that the repulsions are the least, and the distance between them is maximum. • The stable arrangement of the valence electron pairs of atoms helps in determining the molecular geometry Valence shell electrons involved in bonding are known as bonding pairs of electrons (bp), and those valence shell electrons that are not involved in bonding are termed as lone pairs of electrons (lp).
BrCl Geometry
We have to count the total number of valence shell electrons on the central atom. In the case of a diatomic molecule, there is no central atom. Still, we assume the less electronegative and larger one (Br) to be the central atom. The group number of Br is 17, so valence electrons are 7. We add one to the total number of valence shell electrons for each substituent, and the total becomes 8. On dividing 8 by 2, we get 4, which means 4 electron pairs. Out of 4, we have one bond pair of electrons and three lone pairs of electrons. We have A-Br, X-Cl, E- lone pair from the table. We get that molecular shape is linear, and electron geometry is tetrahedral.
BrCl Hybridization
The concept of hybridization explains the geometrical shape and bonding in polyatomic molecules. Hybridization can be defined as mixing pure atomic orbitals to form hybrid atomic orbitals. This mixing is feasible if the pure atomic orbitals have similar shapes and energy. It involves the redistribution of energy when two atomic orbitals combine to form a hybrid orbital. The redistribution of energy takes place between the energy of orbitals of individual atoms to give orbits of equivalent energy. For instance, 2s and one 2p can form sp hybrid orbitals, but 2s and 5d cannot. Also, in C (carbon atom), one 2s and three 2p orbitals are mixed to form 4 equivalent sp3 hybrid orbitals.
The concept of hybridization is a necessity for the explanation of some polyatomic molecules. Many compounds can be explained without the use of hybridization. Some of them are- • Diatomic molecules. For example- HCl • Polyatomic molecules in which the central atom is large enough ( atoms belonging to a third group and above) to accommodate the side atoms ( side atoms should not be much more electronegative than the central atom). This is Drago’s rule. For example PH3 In BrCl, the bond is a sigma bond formed by the overlap of 3p and 4p orbitals of Cl and Br, respectively. There is no need for hybridization to explain this bond. Thus, BrCl is an unhybridized molecule. If a molecule is hybridized, the hybridization can be found out by using the total number of electron pairs, which are found while calculating shape via VSEPR theory. For instance, BrCl had 4 pairs of electrons. In the table, hybridization is sp3 for 4 pairs of electrons. If it had been hybridized, it would be sp3.
BrCl Polarity
The polarity of a compound is decided by the presence or absence of a net dipole moment. Dipole moment depends on the difference in electronegativity of two atoms forming a bond. Here, we have only one bond that is between Br and Cl. Both are highly electronegative, and Cl is more electronegative than Br. The electronegativity of Cl is 3.16, and Br is 2.96.
The shape of the molecule is linear, and there is no other bond that might cancel the dipole moment of this bond. Thus, the polar Br-Cl bond leads to a polar molecule.
Conclusion
BrCl is a yellow gaseous compound at room temperature. It is a polar covalent molecule. In Lewis structure, one bond between two atoms satisfies the octet rule and formal charge. The geometry of BrCl is tetrahedral, and the shape is linear. BrCl is a diatomic and unhybridized molecule. Comment your questions if you have any. Happy Learning!